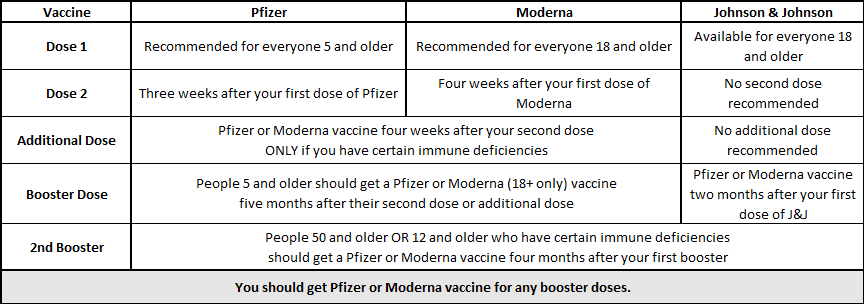
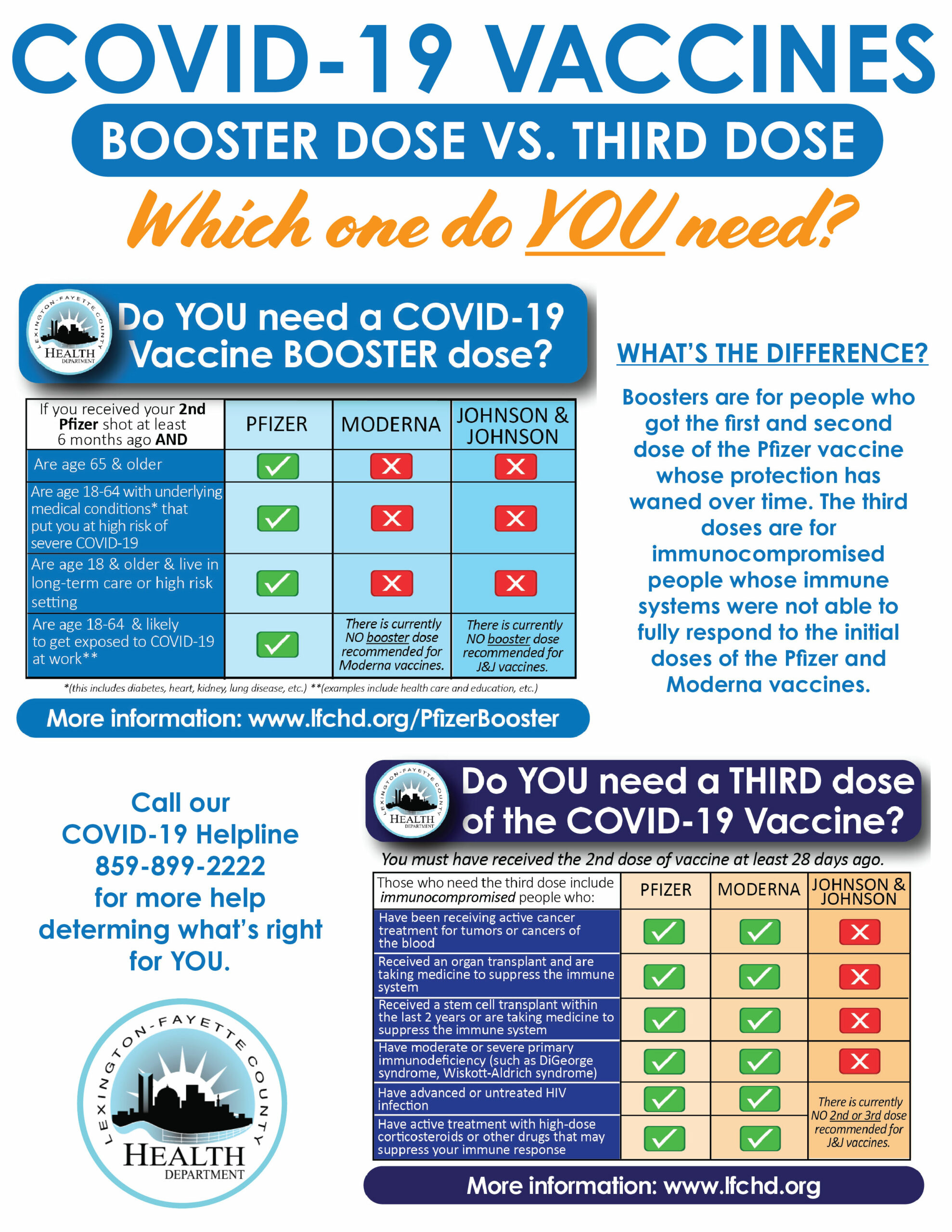
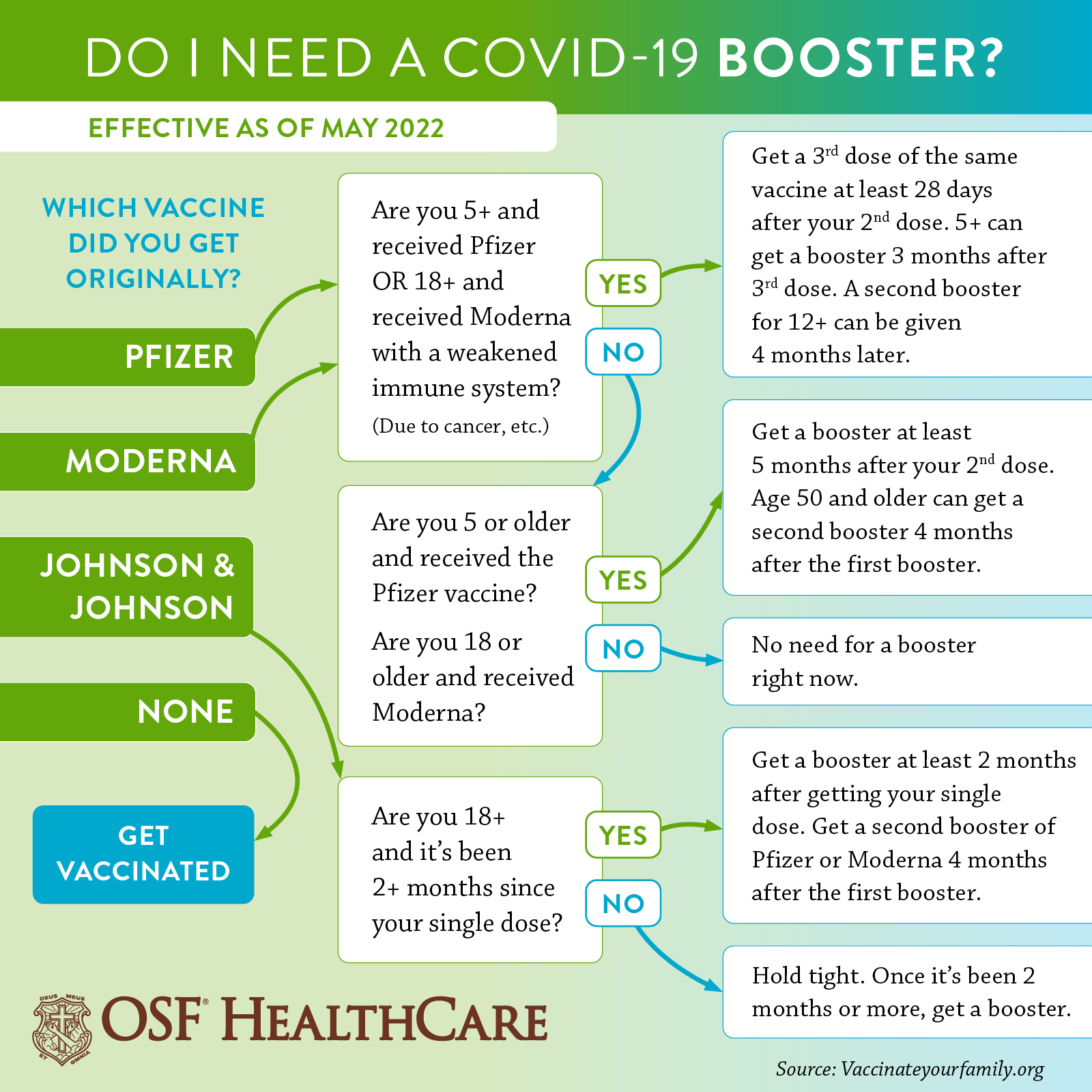
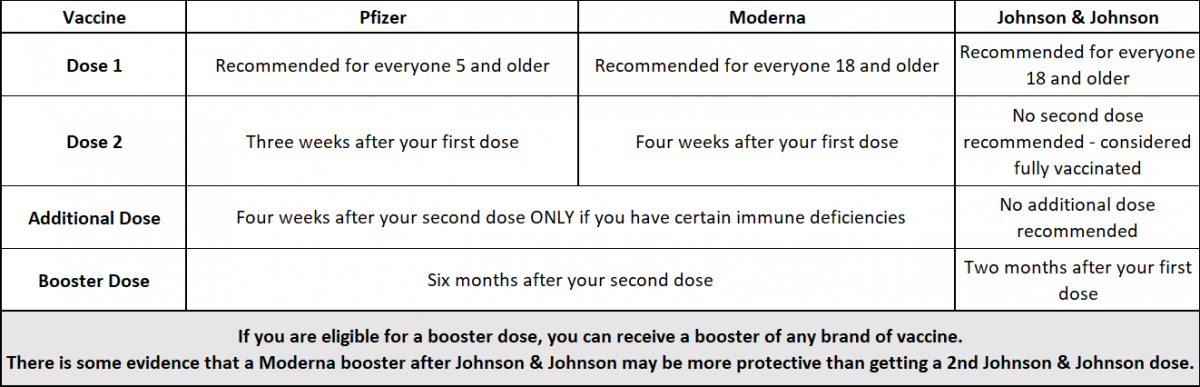
Moderna seeks FDA authorization for a second booster dose of its coronavirus vaccine for all adults - The Washington Post

FDA panel recommends Moderna booster for people 65 and older and adults at high risk of exposure or severe illness - The Washington Post
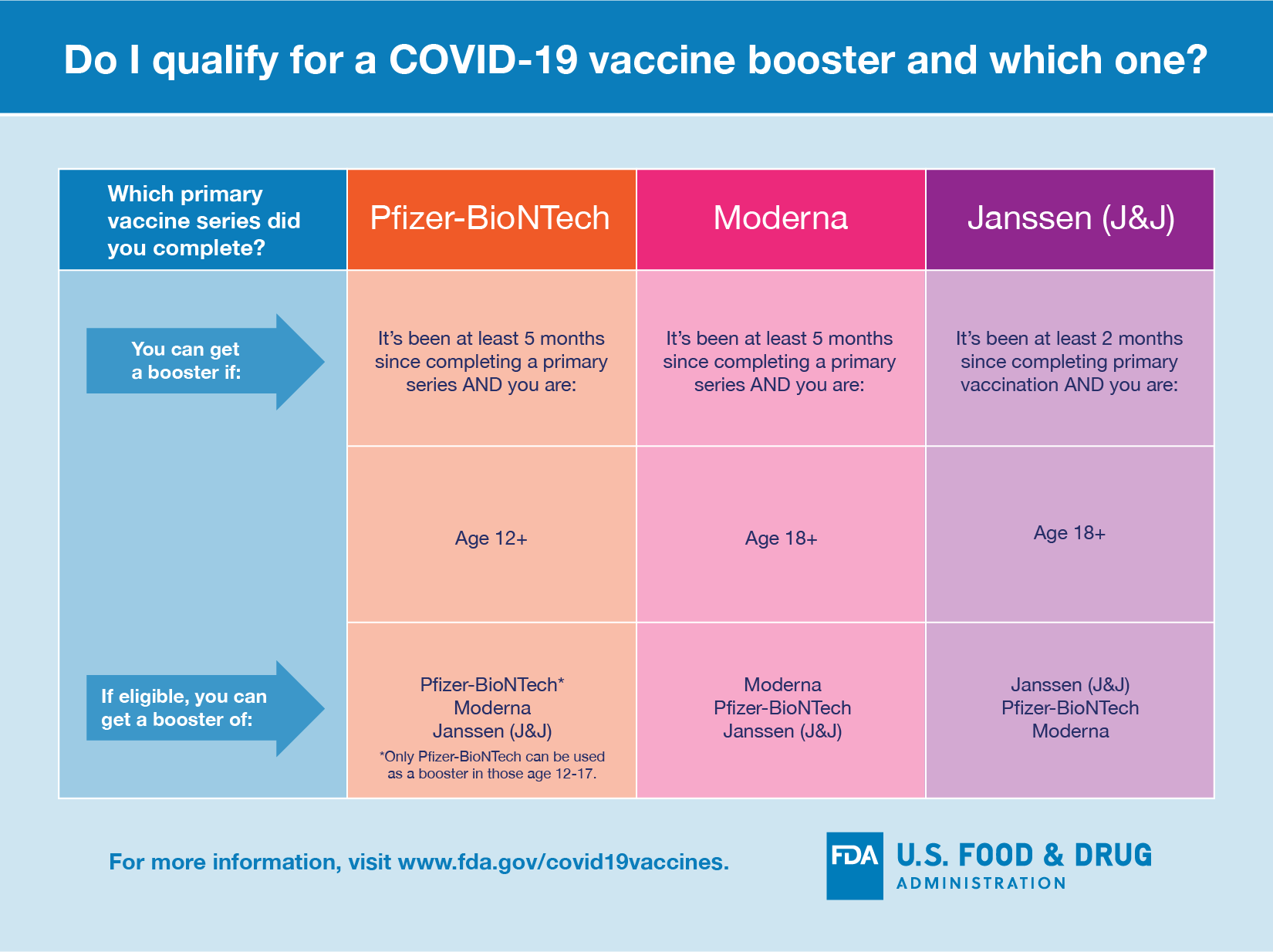
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA