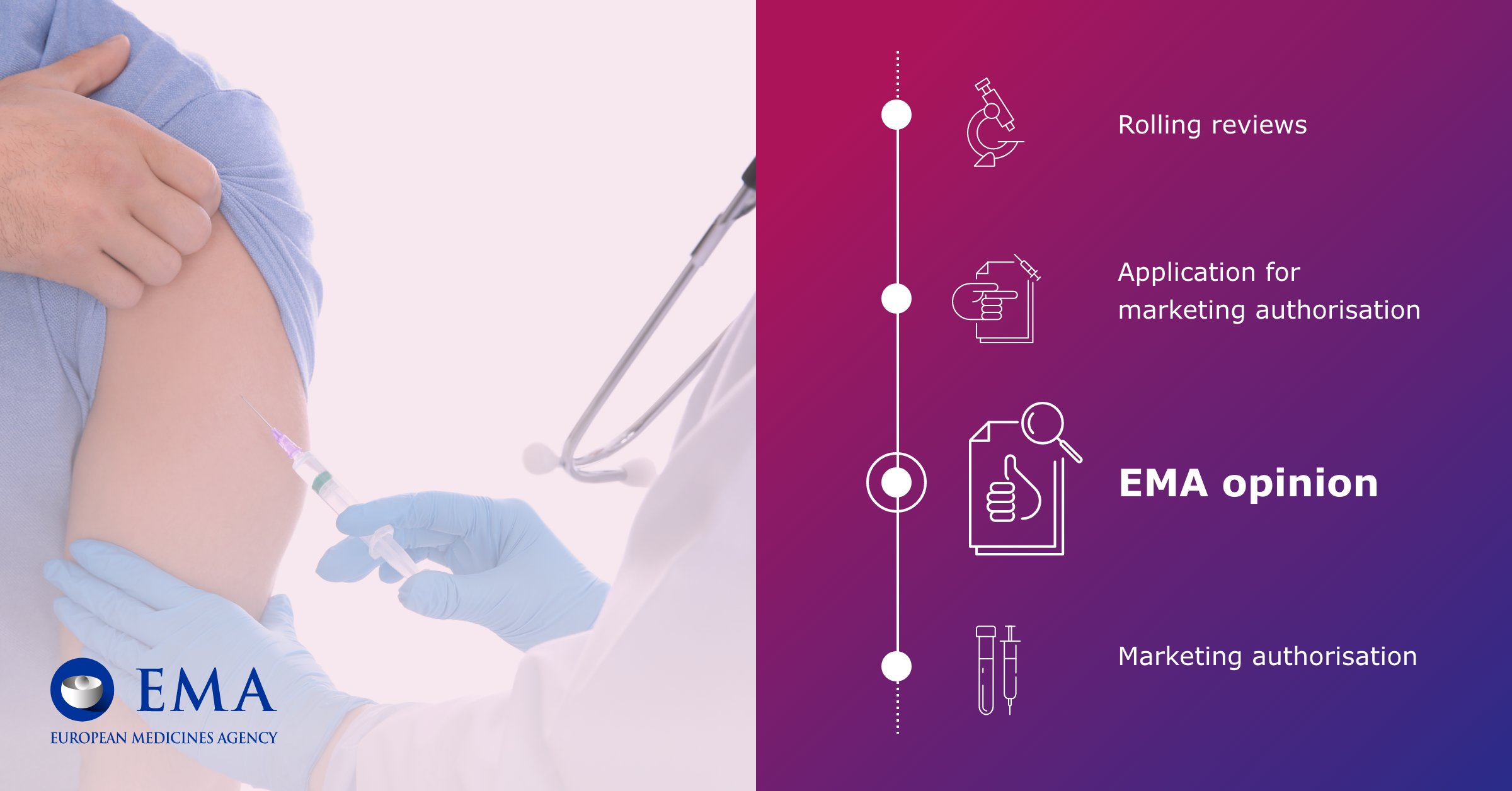
S.E.F.H on X: "💉La @EMA_News recomienda dosis extra de Pfizer o Moderna en personas inmunodeprimidas al menos 28 días después de completar la pauta 💉Además, concluye que se puede administrar una 3ª
La EMA advierte de que "aunque la ola de ómicron parece perder fuerza, tenemos que ser cautos" | Salud
ECDC-EMA statement on booster vaccination with Omicron adapted bivalent COVID-19 vaccines | CDE Almería - Centro de Documentación Europea - Universidad de Almería

EMA evaluating data on booster dose of COVID-19 Vaccine Janssen | CDE Almería - Centro de Documentación Europea - Universidad de Almería

News - The European Commission and the Committee for Medicinal Products for Human Use at the EMA Voted Positively for mRNA Booster Vaccinations from BioNTech/Pfizer and Moderna Adapted to the Omicron Virus